√ダウンロード norfloxacin structure 308369-Norfloxacin structure name
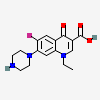
Seven new molecular salts of norfloxacin (1ethyl6fluoro4oxo7piperazin1yl1 H quinoline3carboxylic acid;In oral and intravenous formulations, levofloxacin is indicated in adults for the treatment of various infections caused by susceptible bacteria, including infections of the upper respiratory tract, lower respiratory tract, skin, skin structures, urinary tract, and prostateL,L The oral formulation is also indicated in both adults and children 6 months of age and older for the postThe dihydrate structure has one norfloxacin and two water molecules in the asymmetric unit NF 125 hydrate crystal structure was satisfactorily solved and refined to a good Rfactor of with two crystallographically unique norfloxacin molecules (A and B), two water molecules (O1W, O2W), and a third water (O3W) of 050 site occupancy (see ORTEP in Figure S1a, data
Norfloxacin C16h18fn3o3 Pubchem
Norfloxacin structure name
Norfloxacin structure name-0504 · Recently, the crystal structures of cobalt complexes of gatifloxacin , enrofloxacin , pipemidic acid , and norfloxacin have also been reported Based on the points above, we aimed to modify the surface of POMs with medically relevant molecules to improve their properties During our efforts to modify and functionalize POMs with biologically active molecules, Norfloxacin (NF)The aim of the present paper was to obtain new solid forms of Norfloxacin For this purpose Norfloxacin was recrystallized from acetic acid and its mixtures with several organic solvents By recrystallization of Norfloxacin from acetic acid and from its mixture with several organic solvents a new solvate was found To evidence this new solid form of Norfloxacin different investigation



The Fluoroquinolones Mayo Clinic Proceedings
Abbreviated as NF) with various organic acids (adipic acid, mucic acid, oOHbenzoic acid, mOHbenzoic acid, pOHbenzoic acid, naphthalene1, 5disulfonic acid and naphthalene2sulfonic acid) were synthesized and their crystal structures were determined byEnglish Norfloxacin is a bioactive molecule of 4pyridone group that inhibits bacterial type IIA topoisomerase (DNA gyrase and topoisomerase IV) CAS Reg No InChIKey OGJPXUAPXNRGGIUHFFFAOYSAN Reference Forouzesh, Abed;For this purpose Norfloxacin was recrystallized from acetic acid and its mixtures with several organic solvents By recrystallization of Norfloxacin from acetic acid and from its mixture with several organic solvents a new solvate was found To evidence this new solid form of Norfloxacin different investigation techniques were used powder Xray diffraction, FTIR, DSC,
Norfloxacin is the first antimicrobial in the fluoroquinolone class to be marketed in the United States;New solid form of Norfloxacin Structural studies January 11;A simple, sensitive and reproducible ultraperformance liquid chromatography method for determination of ciprofloxacin, difloxacin, lomefloxacin, norfloxacin and ofloxacin oxidation stability under permanganate treatment in acidic conditions at pH from 30 to 60, was developed Chromatographic sepa
Norfloxacin is a broadspectrum antibiotic that is active against both Grampositive and Gramnegative bacteria, which functions by inhibiting DNA gyrase;Target DNA gyrase;Two novel hydrate forms of norfloxacin (NF) were serendipitously obtained during cocrystallization with eugenol NF 125 hydrate and 1125 hydrate are isomorphous crystal structures in the P21/c space group, each with two symmetryindependent drug molecules, two full water molecules, and a third water of 05 and 025 partial occupancy, respectively Water promoted proton transfer results in a shift from neutral to ionic hydrogen bonding between norfloxacin molecules in hydrate structuresNorfloxacin, a quinolone antibacterial reagent, has been studied with respect to its binding to calf thymus DNA using fluorescence and linear dichroism techniques and unwinding of supercoiled DNA The fluorescence of norfloxacin is strongly quenched in the presence of DNA and using this decrease in a fluorescence titration the equilibrium constant of the complex formation was
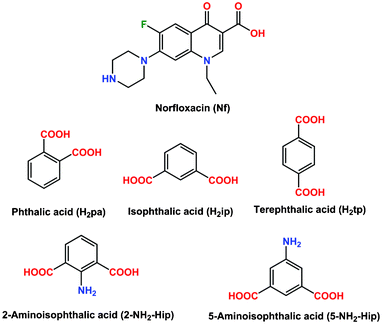


Norfloxacin Salts With Benzenedicarboxylic Acids Charge Assisted Hydrogen Bonding Recognition And Solubility Regulation Crystengcomm Rsc Publishing


Different Conformations Of Norfloxacin In The Crystal Structures Of The Download Scientific Diagram
Structure and biological properties of the copper(II) complex with the quinolone antibacterial drug Npropylnorfloxacin and 2,2'bipyridine Efthimiadou EK(1), Thomadaki H, Sanakis Y, Raptopoulou CP, Katsaros N, Scorilas A, Karaliota A, Psomas G Author information (1)Institute of Physical Chemistry, NCSR Demokritos, GR Aghia Paraskevi Attikis, Greece The neutralNorfloxacin hydrochloride C16H19ClFN3O3 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more · Abstract Alginate–Fe 2 /Fe 3 polymer coated Fe 3 O 4 magnetic nanoparticles (Fe 3 O 4 @ALG/Fe MNPs) with core/shell structure are prepared and used as heterogeneous Fenton nanocatalyst to degrade norfloxacin (NOF) The Fentonlike process based on Fe 3 O 4 @ALG/Fe shows much higher efficiency on NOF degradation


Norfloxacin 96 7
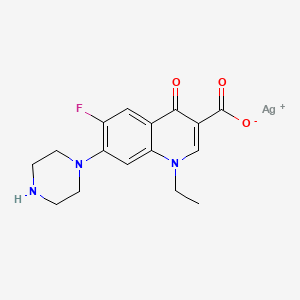


Silver Norfloxacin C16h17agfn3o3 Pubchem
NCI Thesaurus (NCIt) Gatifloxacin is a monocarboxylic acid that is 4oxo1,4dihydroquinoline3carboxylic acid which is substituted on the nitrogen by a cyclopropyl group and at positions 6, 7, and 8 by fluoro, 3methylpiperazin1yl, and methoxy groups, respectively Gatifloxacin is an antibiotic of the fourthgeneration fluoroquinolone family,In this work, novel and efficient norfloxacinmolecularly imprinted composite membranes with an organic–inorganic structure (NFIcMs) were designed and developed using GO/PVDF blended membranes as substrates for selective separation and fast enrichment of norfloxacin31 Crystal structures of ciprofloxacin and norfloxacin fumarates The crystal structures of CIP Fum H 2 O (1 1 1) and CIP Fum H 2 O (1 05 15) have been described in previous works 10,12 The relevant crystallographic data for the previously unknown hydrates of norfloxacin fumarate and the anhydrous ciprofloxacin fumarate salts are given in Table 1


Suprabank Molecules Norfloxacin



Ciprofloxacin Wikipedia
Zand, Eskandar (19) "Reliable target prediction of bioactive moleculesDateiNorfloxacin Structural Formula V1svg Zur Navigation springen Zur Suche springen Datei;In this study, two new antibacterial complexes based on the quinolone antibacterial drugs Norfloxacin (NF) and Ciprofloxacin (CF) and the polyoxometalates (POMs) were synthesized, that is, Co II (C 16 H 18 FN 3 O 3) 2 2 H 4 SiW 12 O 40·6H 2 O (1) and Co II (C 17 H 18 FN 3 O 3) 2 2 H 4 SiW 12 O 40·2H 2 O (2)Then the complexes were comprehensively characterized by
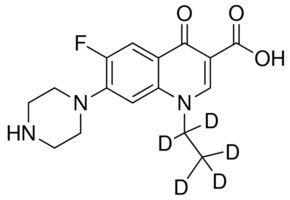


Norfloxacin D5 Vetranal Analytical Standard 57 1 Sigma Aldrich



Pnwh0gddkbbodm
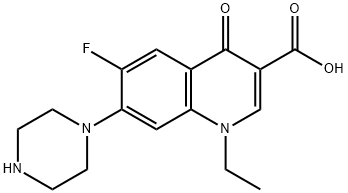
»Norfloxacin contains not less than 990percent and not more than 1010percent of C 16 H 18 FN 3 O 3,calculated on the dried basis Packaging and storage — Preserve in tight,lightresistant containers USP Reference standards á11ñ — USP Norfloxacin RS Identification— A Infrared Absorption á197Mñ B Ultraviolet Absorption á197Uñ —N OTE— Uselow actinic glassware inEidem, US (1978, 1979 both to Kyorin);Norfloxacin is an antibiotic in a group of drugs called fluoroquinolones (floroKWINolones) Norfloxacin fights bacteria in the body Norfloxacin is used to treat different bacterial infections of the prostate or urinary tract (bladder and kidneys) Norfloxacin is also used to treat gonorrhea



Structure Of Norfloxacin Download Scientific Diagram



Norfloxacin Nicotinate Fluoroquinolone Antibiotic Agent Cas 1103 81 9 Ab
Simon, S The aim of the present paper was to obtain new solid forms of Norfloxacin For this purpose Norfloxacin was recrystallized from acetic acid and its mixtures with several organic solvents By recrystallizationStructural Science, Crystal Engineering and Materials ISSN 5256 Supramolecular structures and physicochemical properties of norfloxacin salts Yun Xu, Linglei Jiang and Xuefeng MeiTwo novel hydrate forms of norfloxacin (NF) were serendipitously obtained during cocrystallization with eugenol NF 125 hydrate and 1125 hydrate are isomorphous crystal structures in



Chemical Structures Of Ciprofloxacin And Norfloxacin Download Scientific Diagram
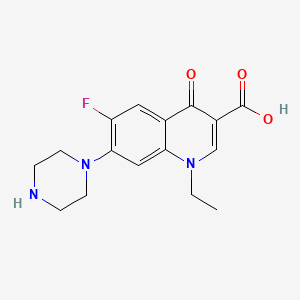


Norfloxacin Drug Information Uses Side Effects Chemistry Pharmacompass Com
Norfloxacin Chemical structures of the synthesized polymers have been confirmed by 1H and 13C solution NMR as well as FTIR spectroscopy Absolute molecular weights of polymers have been determined using gel permeation chromatography (GPC), endgroup analysis and matrixassisted laser desorption/ionization timeofflight mass spectrometry (MALDITOF MS) Results · Structural differences among fluoroquinolones, notably overall molecular hydrophobicity and bulkiness of the C7 substituent, on the invitro susceptibilities of fluoroquinoloneresistant strains of Streptococcus pneumoniae to norfloxacin, J Antimicrob Chemother, 1997, vol 40 (pg ) Google Scholar Crossref Search ADS PubMed 27 GillNorfloxacin is a quinolone/fluoroquinolone antibiotic Norfloxacin is bactericidal and its mode of action depends on blocking of bacterial DNA replication by binding itself to an enzyme called DNA gyrase, which allows the untwisting required to replicate one DNA double helix into two Notably the drug has 100 times higher affinity for bacterial DNA gyrase than for mammalian



Figure 1 From A Novel Biochar Derived From Cauliflower Brassica Oleracea L Roots Could Remove Norfloxacin And Chlortetracycline Efficiently Semantic Scholar


X5x8rc9brsrlxm
Synthesis, spectroscopic, structure, thermal analyses, and biological activity evaluation of new norfloxacin vanadium (V) solvates (L) (L = An, DMF, Py, Et3N and oTol) Article Fulltext availableOfloxacin, like other 4quinolones, is unusual among front line drugs available to treat bacterial infections since it affects bacterial DNA synthesis, rather than cell wall or protein synthesis 2Ofloxacin ( mg/kg), norfloxacin (40 mg/kg), pefloxacin mesylate dihydrate (40 mg/kg)and ciprofloxacin (50 mg/kg) were administered by gavage twice daily for three consecutive weeks 6Spectroscopy 25(1) DOI /SPE Authors Ioan Bratu National Institute for



Different Types Of Copper Complexes With The Quinolone Antimicrobial Drugs Ofloxacin And Norfloxacin Structure Dna And Albumin Binding Sciencedirect


Norfloxacin C16h18fn3o3 Chemspider
AntibacterialNorfloxacin is a synthetic chemotherapeutic antibacterial agent occasionally used to treat common as well as complicated urinary tract infections Norfloxacin is a broadspectrum antibiotic that is active against both Grampositive and Gramnegative bacteria · Adsorption of antibiotics on solid particles is a key process controlling their fate in the subsurface This study compared the adsorption of ofloxacin and norfloxacin (NOR) on carbon nanotubes (CNTs) to evaluate the role of structural and hydrophobic properties in regulating their adsorption A significant relationship was observed between singlepoint adsorption coefficientsA synthetic fluoroquinolone (FLUOROQUINOLONES) with broadspectrum antibacterial activity against most gramnegative and grampositive bacteria Norfloxacin inhibits bacterial DNA



Figure 1 From Uranium Vi And Zirconium Iv Of The Second Generation Quinolone Antimicrobial Drug Norfloxacin Structure And Biological Activity Semantic Scholar
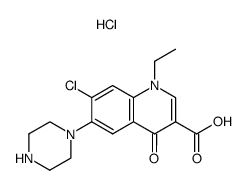


Cas 77 3 Norfloxacin Ep Impurity B Norfloxacin Ethylenediamine Analog 7 2 Aminoethyl Amino 1 Ethyl 6 Fluoro 4 Oxo 1 4 Dihydroquinoline 3 Carboxylic Acid Hcl Chemsrc
Seven new molecular salts of norfloxacin (1ethyl6fluoro4oxo7piperazin1yl1Hquinoline3carboxylic acid;M Pesson, DE ;Ciprofloxacin is under investigation in clinical trials The fluoroquinolones are structurally related to nalidixic acid The activity and spectrum are enhanced by the addition of 6fluoro and 7piperazino substituents Quinolone antimicrobials appear to inhibit DNA gyrase, an enzyme


Norfloxacin C16h18fn3o3 Pubchem


Quinolones Infectious Disease And Antimicrobial Agents
Abbreviated as NF) with various organic acids (adipic acid, mucic acid, o OHGröße der PNGVorschau dieser SVGDatei 454 × 258 Pixel Weitere Auflösungen 3 · Two novel hydrate forms of norfloxacin (NF) were serendipitously obtained during cocrystallization with eugenol NF 125 hydrate and 1125 hydrate are isomorphous crystal structures in the P21/c space group, each with two symmetryindependent drug molecules, two full water molecules, and a third water of 05 and 025 partial occupancy, respectively



Figure 1 From Fluorescence Studies Of Gold Iii Norfloxacin Complexes In Aqueous Solutions Semantic Scholar


Quinolones Infectious Disease And Antimicrobial Agents
· It is also known that norfloxacin can act as a protonated counter cation in structures as in (nfH 2)(nfH)CuCl 4Cl·H 2 O and (nfH 2)(nfH)ZnCl 4Cl·H 2 O ; · This study compared the adsorption of ofloxacin and norfloxacin (NOR) on carbon nanotubes (CNTs) to evaluate the role of structural and hydrophobic properties in regulating their adsorption A significant relationship was observed between singlepoint adsorption coefficients ( K d ) and specific surface area (highly hydrophobic), but not between K d s and oxygen contentMolecular Formula C22H23FN4O5 Synonyms Norfloxacin nicotinate 1Ethyl6fluoro4oxo7 (piperazin1yl)1,4dihydroquinoline3carboxylic acid compound with nicotinic acid (11) C22H23FN4O5 1ethyl6fluoro4oxo7piperazin1ylquinoline3carboxylic acid;pyridine3carboxylic acid



Norfloxacin Nicotinate Cas 1103 81 9 Glentham Life Sciences



Structures Of Ciprofloxacin Norfloxacin And Ofloxacin Dashed Box Download Scientific Diagram
Synthesis and structural analysis of polyester prodrugs of norfloxacin Molecules 08 Jan 18;13(1) doi /molecules Authors Marcin SobczakLiterature References Fluorinated quinolone antibacterial Prepn T Irikura, BE ;New solid form of Norfloxacin Structural studies Bratu, I ;


Desethylene Norfloxacin Hydrochloride 77 3
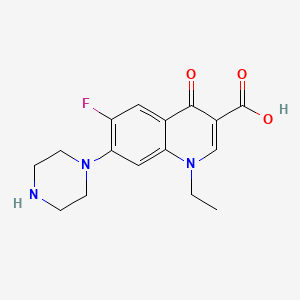


Norfloxacin C16h18fn3o3 Pubchem
Diversity of crystal structures and physicochemical properties of ciprofloxacin and norfloxacin salts with fumaric acid† Artem O Surov, a Alexander P Voronin, a Ksenia V Drozd, a Andrei V Churakov,b Pascal Roussel c and German L Perlovich *ad The crystallization of norfloxacin and ciprofloxacin – antibacterial fluoroquinolone compounds – with fumaric acid resulted in theIn these complexes no direct coordination between metal and quinolone is observedNorfloxacin (MK0366) is a synthetic chemotherapeutic antibacterial agent occasionally used to treat common as well as complicated urinary tract infections Norfloxacin (MK0366) is a broadspectrum antibiotic that is active against both Grampositive and Gramnegative bacteria It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV, enzymes


Norfloxacin Wikidoc
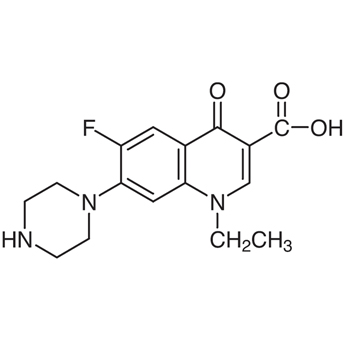


Norfloxacin 96 7 Tokyo Chemical Industry Co Ltd Apac



Multidrug Salt Forms Of Norfloxacin With Non Steroidal Anti Inflammatory Drugs Solubility And Membrane Permeability Studies Crystengcomm Rsc Publishing



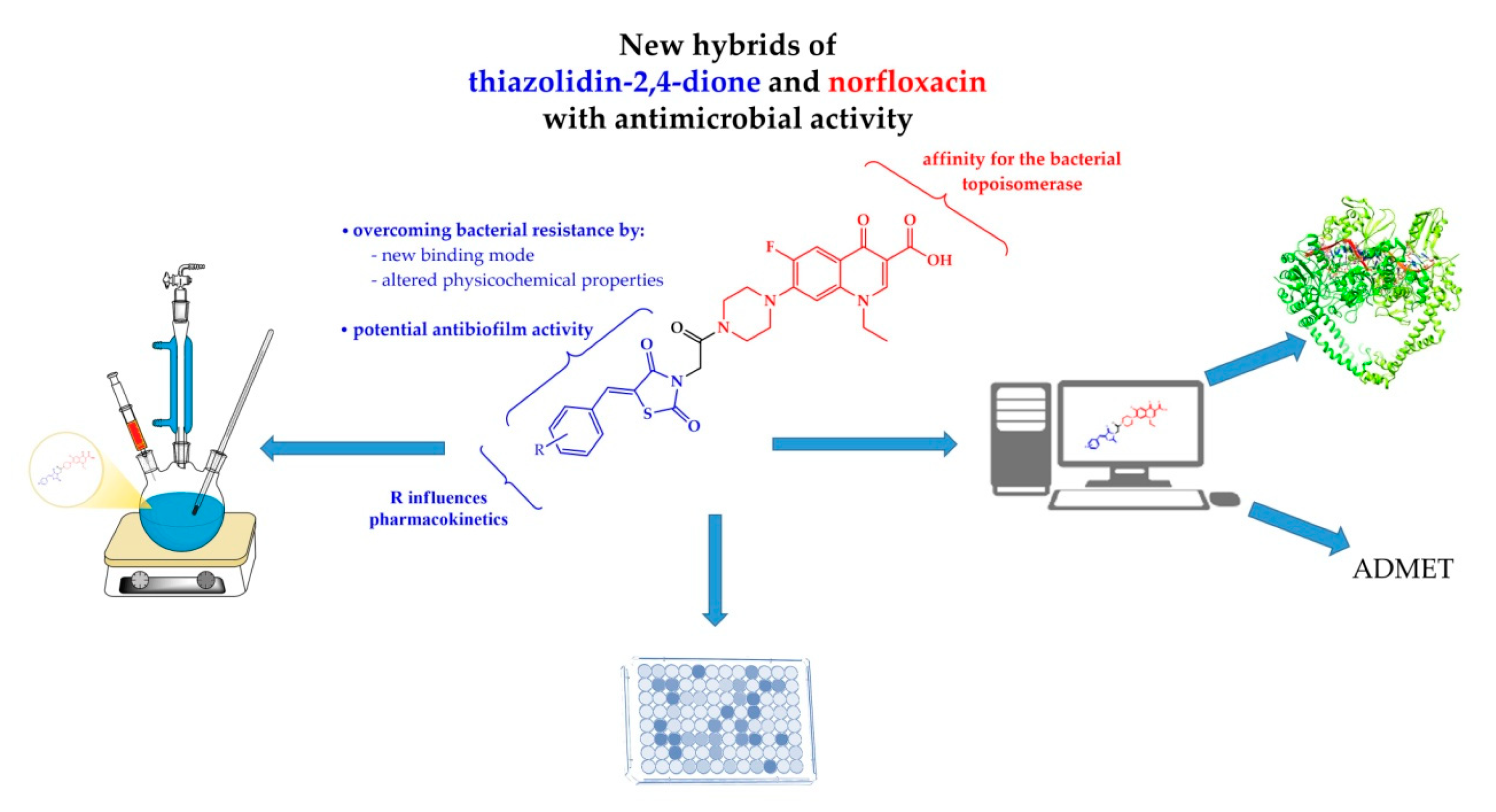
Design And Synthesis Of New Norfloxacin 1 3 4 Oxadiazole Hybrids As Antibacterial Agents Against Methicillin Resistant Staphylococcus Aureus Mrsa Sciencedirect


Ofloxacin Wikipedia



The Fluoroquinolones Mayo Clinic Proceedings


Structures Of Norfloxacin 1 And Levofloxacin 2 Download Scientific Diagram



Chemical Structure Of Norfloxacin Download Scientific Diagram



Chemical Structures Of The Antibacterial Drug Norfloxacin And Download Scientific Diagram



File Norfloxacin Structure Svg Wikipedia


Norfloxacin Chemical Structure Molecular Formula Reference Standards


Norfloxacin
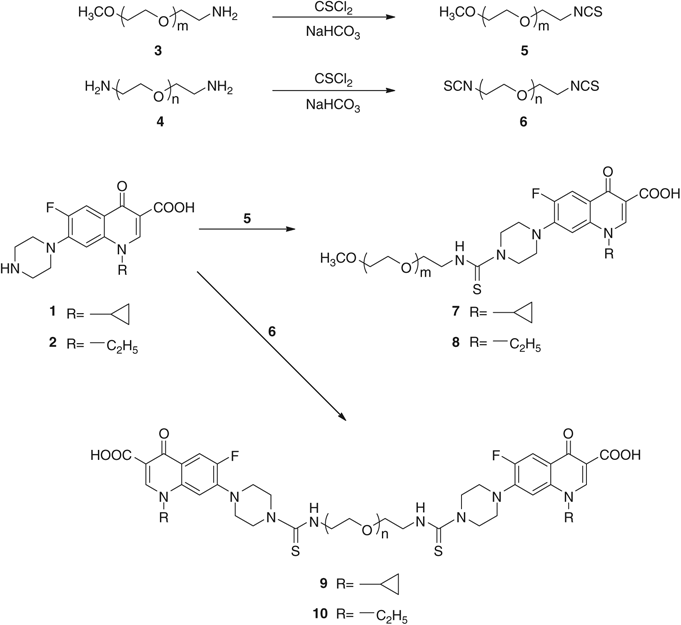


Synthesis And Antimicrobial Activity Of Ciprofloxacin And Norfloxacin Permanently Bonded To Polyethylene Glycol By A Thiourea Linker The Journal Of Antibiotics
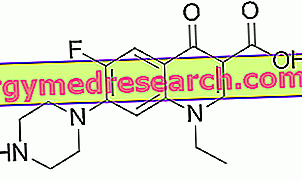


Norfloxacin Drugs 21
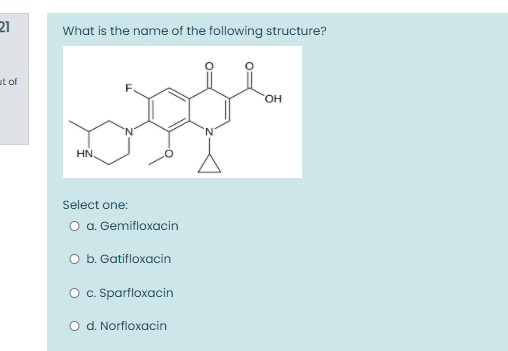


Solved 21 What Is The Name Of The Following Structure It Chegg Com
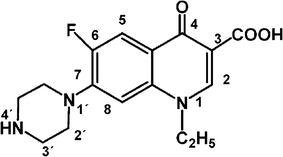


Interaction Of The Antibiotic Norfloxacin With Ionic Micelles Ph Dependent Binding Springerlink
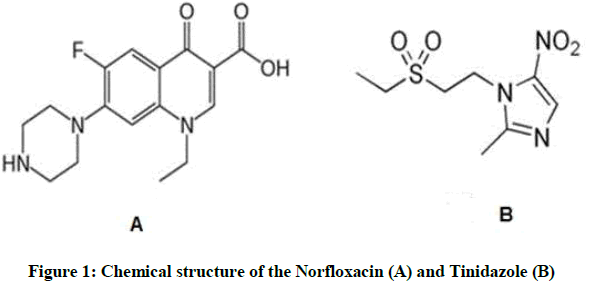


Analytical Application Of Different Spectrophotometric Methods For Simultaneous Determination Of Norfloxacin And Tinidazole In Their Pure Forms And Their Pharmaceutical Preparation


Norfloxacin Glucuronate C22h28fn3o10 Pubchem



Figure 1 Synthesis Characterization And Antifungal Studies Of Cr Iii Complex Of Norfloxacin And Bipiridyl Ligand



Chemical Structures Of Norfloxacin Nor Lomefloxacin Lom Download Scientific Diagram


Development Of An Immunoenzyme Assay To Control The Total Content Of Antibiotics Of The Fluoroquinolone Group In Milk Springerlink



Norfloxacin Hydrochloride 93 0 Carbosynth Product


Apexbio Norfloxacin Hydrochloride
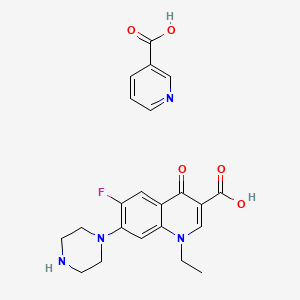


Norfloxacin Nicotinate C22h23fn4o5 Pubchem


Norfloxacin Atr Ir Spectrum Spectrabase
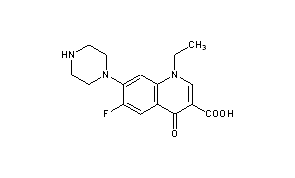


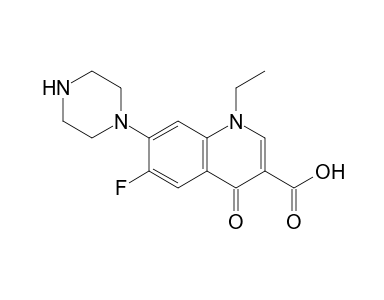
Norfloxacin



Molecular Structures Of Ofloxacin Ofx Norfloxacin Nofx Download Scientific Diagram
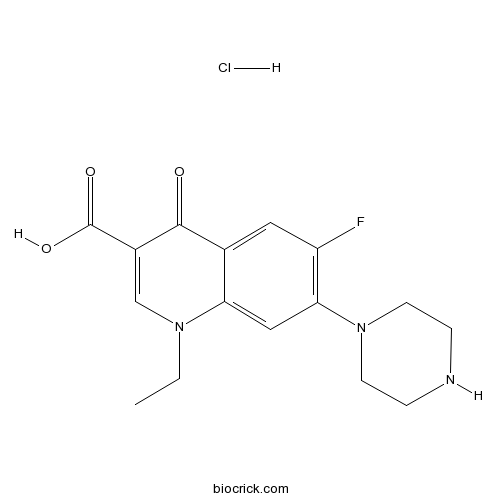


Norfloxacin Hydrochloride Cas 27 0 High Purity Manufacturer Biocrick
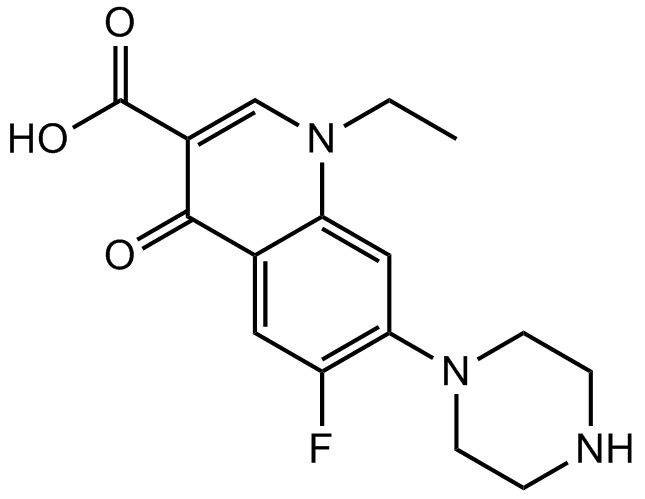


Apexbio Norfloxacin


Antibiotic Drugs Information Description On Norfloxacin Eye Ear Drop



Chemical Structure Of Norfloxacin Cas 7 Download Scientific Diagram



Characterization Of Mucoadhesive Norfloxacin Suspensions By Fourier Transform Infrared Spectroscopy Semantic Scholar



The Chemical Structure Of 1 Ciprofloxacin 2 Norfloxacin 3 Download Scientific Diagram



Norfloxacin C16h18fn3o3 Pubchem
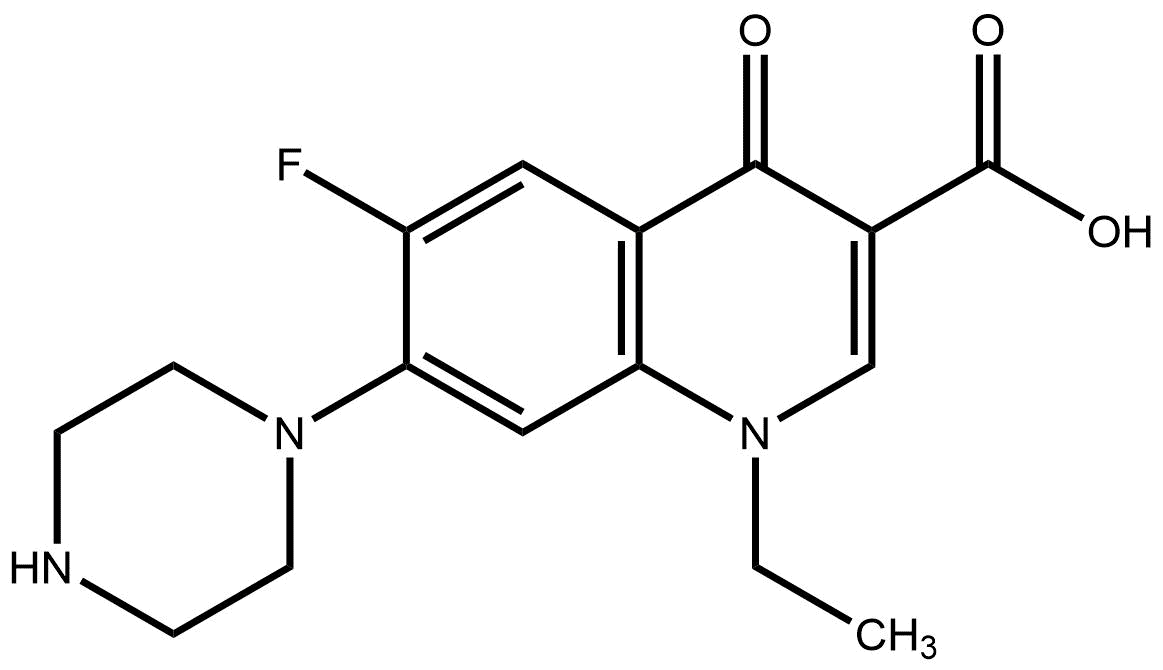


Lepu Medical Antibiotics Norfloxacin Pharmaceuticals Medicine Drugs Manufacturer
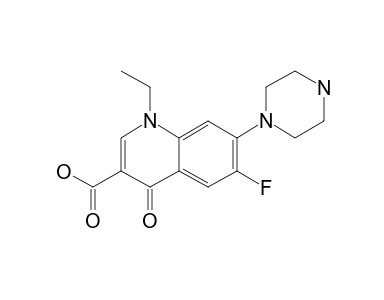


Norfloxacin 13c Nmr Chemical Shifts Spectrabase



Norfloxacin Structure C16h18fn3o3 Over 100 Million Chemical Compounds Mol Instincts
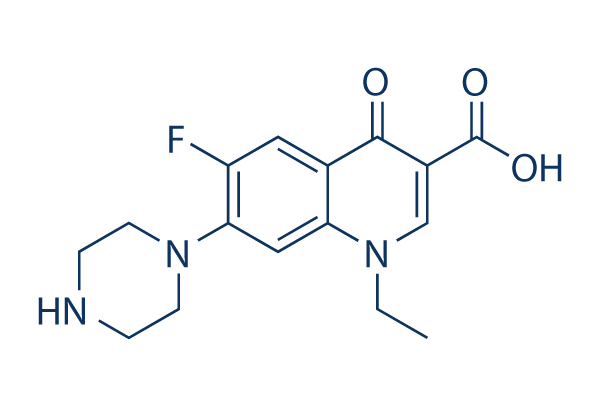


Norfloxacin 99 Hplc Selleck Topoisomerase Inhibitor Qcfile



Iucr Supramolecular Structures And Physicochemical Properties Of Norfloxacin Salts
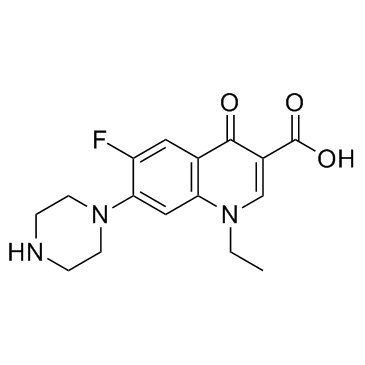


Norfloxacin Cas 96 7 Chemsrc


Norfloxacin Ep Impurity E 78 4



Norfloxacin Search Results Mybiosource



Design And Synthesis Of Aminothiazolyl Norfloxacin Analogues As Potential Antimicrobial Agents And Their Biological Evaluation Sciencedirect



27 3 N 3 Oxobutyl Norfloxacin 1 Ethyl 6 Fluoro 1 4 Dihydro 4 Oxo 7 4 3 Oxobutyl 1 Piperazinyl 3 Quinolinecarboxylic Acid C H Fn O Trc



Norfloxacin An Overview Sciencedirect Topics



Pdf Uranium Vi And Zirconium Iv Of The Second Generation Quinolone Antimicrobial Drug Norfloxacin Structure And Biological Activity Semantic Scholar


Antibiotic Drugs Information Description On Norfloxacin



Fluoroquinolone Antimicrobial Agents Nejm



Norfloxacin For System Suitability European Pharmacopoeia Ep Reference Standard 96 7 Sigma Aldrich



Norfloxacin



Formulation Of Improved Norfloxacin Hcl Tablets Quality Control Assessment And Comparison Study Of Acidic And Basic Form Of Norfloxacin In Tablet Formulation Semantic Scholar
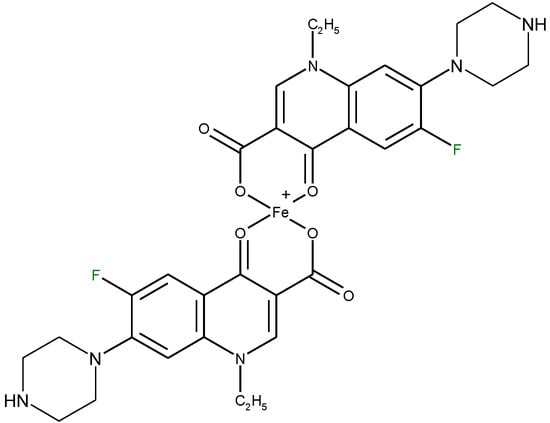


Molecules Free Full Text Metal Complexes Of Quinolone Antibiotics And Their Applications An Update Html
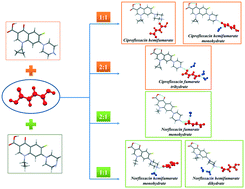


Diversity Of Crystal Structures And Physicochemical Properties Of Ciprofloxacin And Norfloxacin Salts With Fumaric Acid Crystengcomm Rsc Publishing



Structures Of A Representative Fluoroquinolones Enrofloxacin 1 Download Scientific Diagram



Evaluation Of Photoassisted Treatments For Norfloxacin Removal In Water Using Mesoporous Fe2o3 Tio2 Materials Journal Of Environmental Management X Mol
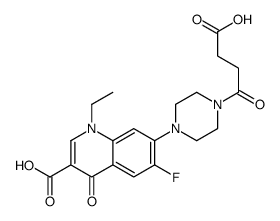


Norfloxacin Succinil Cas 52 8 Chemsrc


Norfloxacin



96 7 Norfloxacin Alb Technology Limited



Structural Insight Into A Quinolone Topoisomerase Ii Dna Complex Journal Of Biological Chemistry


Chemidplus 96 7 Ogjpxuapxnrggi Uhfffaoysa N Norfloxacin Usan Usp Inn Ban Jan Similar Structures Search Synonyms Formulas Resource Links And Other Chemical Information


Quantitative Determination Of Ciprofloxacin And Norfloxacin In Pharmaceutical Preparations By High Performance Liquid Chromatography



86 9 Desethylene Norfloxacin Hydrochloride 7 2 Aminoethyl Amino 1 Ethyl 6 Fluoro 1 4 Dihydro 4 Oxo 3 Quinolinecarboxylic Acid Norfloxacin Ep Impurity B C H Clfn O Trc
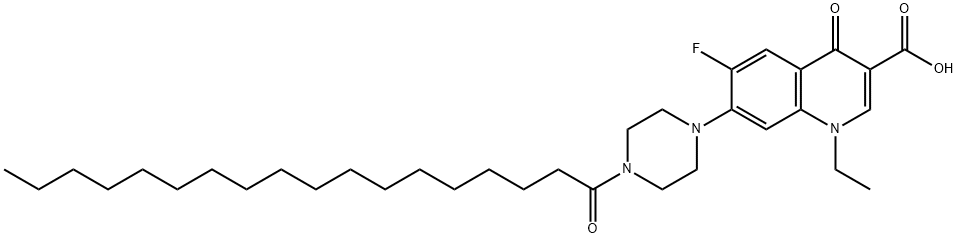


Norfloxacin Imp Ep 08 4



Noroxin Norfloxacin Uses Dosage Side Effects Interactions Warning



Norfloxacin Png Images Pngwing



Norfloxacin Wikipedia



Norfloxacin Wikipedia



Bioequivalence Of Norfloxacin By Hplc Uv Method
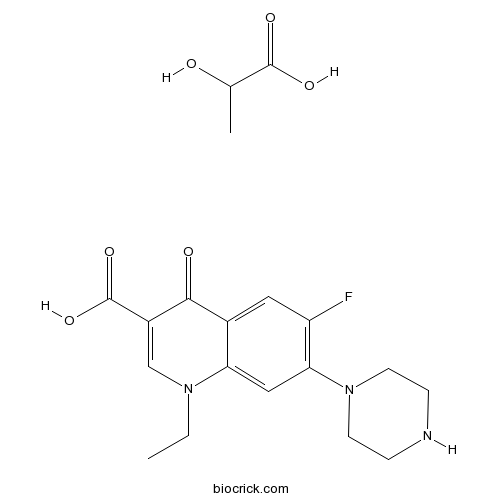


Norfloxacin Lactate Cas 34 0 High Purity Manufacturer Biocrick



Structure Of Norfloxacin Download Scientific Diagram
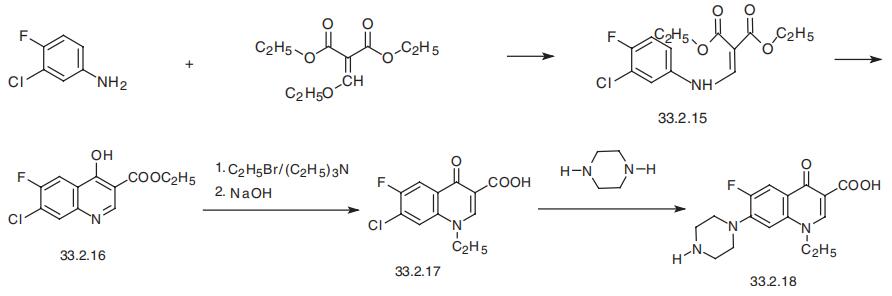


Norfloxacin 96 7
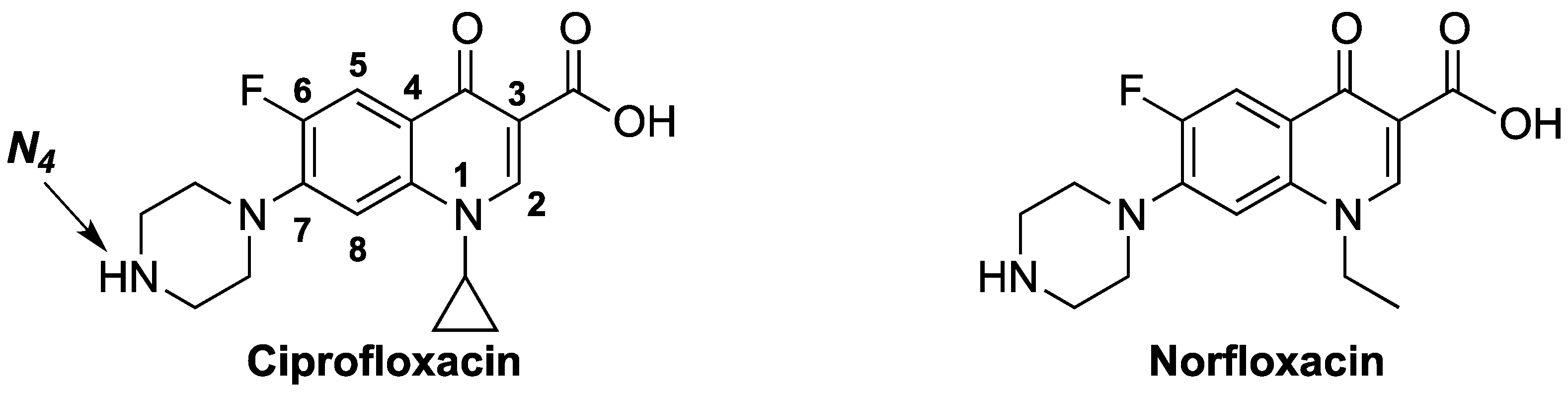


Antibiotics Free Full Text Synthesis Antibacterial Evaluation And Qsar Of A Substituted N4 Acetamides Of Ciprofloxacin And Norfloxacin Html


0054pe


コメント
コメントを投稿